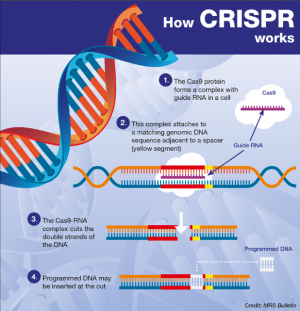
Embryonic Genomic Engineering
Some questions of debate surrounding the subject today include:
- How far can new genomic engineering technology be taken?
- Is it acceptable to allow parents to select characteristics for their children?
- Is it permissible to edit genomes in order to make an individual resistant to a disease?
- Given that some of these editing processes occur during the embryonic stage of a fetus, is it acceptable to carry out these procedures in the absence of consent from the individual being edited?
Contents
History
1953 - 1970s
The origins of genomic editing can be traced back to the discovery of DNA. James Watson and Francis Crick discovered the double helix structure of what is now known as DNA in 1953. This structure was determined using X-Ray diffraction imaging [2]. Werner Arber was the first to discover restriction enzymes in 1968. Arber discovered that restriction enzymes are responsible for cleaving DNA at specific sequences in enzymes isolated from EColi. Researchers used restriction enzymes to expand the field of genomic editing in 1970. This process entailed creating recombinant DNA, inserting it into a host cell, and watching the new genetic material be successfully incorporated and expressed[3].
More Recently
Many advances have occurred over the years, bringing us to where we are today in the field of genomic editing. There are several genome editing technologies available, including ZFNs, TALENs, and CRISPR-Cas9 [4]. CRISPR-Cas9 has been the most popular genetic engineering method since its discovery due to its higher precision rates and self efficiency when compared to other methods. CRISPR principles were discovered by researcher Francisco Mojica in 1993. In the discovery of this locus, “this finding led him to hypothesize, correctly, that CRISPR is an adaptive immune system” laying the groundwork for today's genomic editing technologies [5]. Following this, by the 2000s the necessary technology had been developed and the human genome was sequenced allowing for gene editing technologies to be researched more efficiently. Around 2012 to 2013, researchers such as Virginijus Siksnys and Feng Zhang discovered that CRISPR-Cas9 could be used as a genetic engineering tool to target specific genes in the human genome [6]. CRISPR-Cas9 is now being used in embryonic gene editing and in clinical settings for targeted therapies. The field of genomic editing is still in its early stages, but with the advancement of healthcare and computational technologies, it is projected to develop further.
Types of Genomic Editing
Somatic Gene Editing
Non-reproductive cells in the body are changed by somatic editing. This method only targets a specific gene in a single cell type. Changing this gene has no effect on other cells in the individuals' bodies. After somatic editing, the edited gene will remain within this individual and will not affect the genes of their future children[7][8].
Germline Gene Editing
Germline editing is done on "early-stage embryos, gametes (eggs and sperm), or germ cells that are the precursors of gametes" [9]. As a result of this, every cell in the embryo contains a copy of the edited gene. After this editing has occurred, there is the potential that the edited gene will be passed down to future generations. This is due to the fact that every copy in the embryo contains the edited gene, even sperm and egg cells [10].

Germline vs Somatic Gene Editing in a Clinical Setting
Germline genome editing processes are becoming more accessible in clinical settings than they were previously. Somatic genetic editing has already been approved in a number of clinical settings, making human trials with targeted therapies possible. In these procedures, the editing is more controlled and the effects are restricted to the individuals being edited. Germline editing, on the other hand, has more unknown effects, increased factors, and more unethical implications. Since every cell in the individual has this edited mutation, it is difficult to determine what other unintended effects this induced change may have had. Currently, “any clinical trial proposals for germline alterations will be rejected by the Recombinant DNA Advisory Committee (RAC) of the NIH” [11]. Opposition to these laws is punishable by a fine, imprisonment, or both. In 2019, Jiankui He, a Chinese researcher, went against these guidelines to perform germline genomic experimentation. Jiankui used CRISPR technology to cause embryonic genetic changes that removed HIV-causing genes. His experiment was successful, but the ethical implications of using genomic editing technologies to interfere with human reproduction were controversial as “going against rules and doing such research is forbidden by the international biomedical community” [12]. Although determined unethical, the results of this experiment showed implications for the impacts of germline editing in a clinical setting.
Ethical Implications
Side Effects
An ethical concern with germline genome editing is the potential consequences of inducing an edit without knowing the full extent of the effects. By doing so while being unaware of the consequences, the individual may end up with other complications later in life or complications in future generations of their children. With the technology and knowledge available today, scientists are unable to comprehend the scope of all the unknown side effects of germline genome editing. This lack of understanding can cause modifications that were not intended to happen. One approach is to weigh the consequences of performing the editing procedure versus not performing it. Essentially, the question is whether the benefits of genetic engineering outweigh the risks and uncertainties of "exposing a child to the risks and uncertainties" [13]. Nuclear genome transfers are an example of where the benefits may be deemed to outweigh the risks by policy makers. This eliminates mitochondrial mutations inherited from the maternal parent. If this is not done and the mutation is inherited, the individual is at risk of suffering life-threatening consequences [14]. This criterion is a consideration in germline editing research.
Moral Concerns
An ethical concern is that these trials would be performed on embryos with the potential to develop into human beings. The question here is determining the embryo's moral standing. Individuals debate whether the embryo should be regarded in the same way that we regard a human being or if they should be regarded as merely a collection of cells. If considered as a human being, then conducting experiments in which the genome is altered while ignoring the consequences would be considered unethical. In contrast, if they are considered cells, it is equivalent to experimenting on any human tissue cell; a process that occurs all the time. At this time, there is no agreement on where the moral standing should be. One alternative solution would be to use non-viable embryos for research purposes. For context, a non-viable embryo is one that would not fully adhere to the uterus and correctly develop. However, there are drawbacks to this approach, as the research findings will not be as easily translated for use on a viable embryo[15].
Accessibility
An ethical issue is one of accessibility in genomic editing. Currently, genetic therapies range between $373,000 and $2.1 million in cost [16]. These therapies provide treatments for diseases that are genetically based. The cost of these therapies limit the individuals who are able to have access to them. This creates an economical divide in the healthcare system comparing those who are able to access genomic editing technologies versus those who can not.
Eugenics
Definition
Eugenics is the belief that, as a society, we can improve the quality of the human race by controlling hereditary factors [17]. This term was coined in the late nineteenth century by Francis Galton and was used to justify "morally reprehensible acts" [18]. Genes thought to be harmful would be removed, while those thought to be beneficial would be enhanced. Individuals with disabilities or heritable diseases would have their genes removed from the gene pool, preventing their proliferation. This eliminates variability in the genomic diversity of the human race, resulting in a society with few genetic differences.
Implications Today
As CRISPR-Cas9 and other genomic technologies advance, the debate surrounding eugenics has resurfaced in popularity. There is no formal definition for what is considered the "best" quality of life or what genes are considered to be "good" or "bad". This disagreement can have problematic implications as it entails objectively ranking and labeling individuals' characteristics. Preimplantation genetic diagnosis is one area where eugenics and its complications are becoming more prevalent. This is the concept that embryos can be created and edited before implantation using in vitro fertilization [19]. Embryos with genetic defects would be eliminated in a eugenics context. If used in this sense, this type of genomic editing could infringe on reproductive freedom.
History Repetition
During Galton's reign, the concept of eugenics wielded enormous power in its ability to genetically control the human population. The Nazi Aktion T4 program, which was responsible for the murder of over 70,000 disabled people in order to eliminate those who were deemed "weaker" than others in the human race, is an example of the negative consequences of eugenics. Eugenics was used to justify acts like this and others with moral implications. These historical events and their moral lessons are being remembered as genomic technologies become more common in the medical field [20].
Cognitive Enhancement
Socioeconomic concerns
With germline editing, scientists have the potential to edit aspects of human beings that aren't physical. Currently, scientists have probabilistic arguments for where specific cognitive traits of individuals reside within their DNA [21]. As gene editing becomes more common, there is more discussion about cognitive enhancement. This process creates individuals that are considered to have high cognitive standings within the human race. This can create a general stigma of society becoming less accepting of people who are different widening the gap of socioeconomic statuses [22].
Psychological Traits
Psychological characteristics are influenced by both genetic and environmental factors. These hereditary characteristics can be altered by using germline genome editing. One approach of this argument is to look at these characteristics through the lens of evolution. This viewpoint ignores the possibility that traits have negative or positive connotations and instead examines them in a broader context. This examines whether a trait would have a positive or negative impact on humanity's well-being. Psychopathy, for example, is a cognitive trait that generally has an adverse influence on an individual's role in society. These people are considered dangerous because they have no understanding of the pain they inflict on others [23]. Since this trait is heritable, it may be acceptable to change in genomic editing from this perspective. However, these individuals contribute to genetic diversity, and removing this trait entirely would reduce genetic diversity and have unknown consequences[24].
Cognitive Diversity
Cognitive diversity is regarded as advantageous by many organizations or power structures in society. Having individuals with this diversity can "enable the synthesis of different knowledge bases, perspectives, and opinions necessary to solve difficult problems"[25]. This cognitive diversity has been shown to be beneficial for short-term problem solving. However, research on long-term problem-solving has not been conducted. An example of short-term problem solving would be team projects in an office setting. Cognitive diversity has been shown to have overall positive effects, so the threat of gene editing to its survival raises concerns[26].
Applications
Cardiovascular Disease
Germline genome editing has the potential to improve cardiovascular disease prevention. Hypertrophic cardiomyopathy, for example, is a heritable heart disease. Oregon Health & Science University researchers successfully created gametes with a mutation that causes hypertrophic cardiomyopathy. Following their creation, the scientists corrected the mutation they had caused in order to produce healthy gametes capable of surviving implantation and fertilization[27]. This ideology can be applied to other heritable diseases.
Infertility Treatment
A potential application is infertility treatment using germline genome editing. Scientists can extract sperm cells and edit them to include programmable nucleases, consequently eliminating the mutation that causes infertility. These newly edited cells can then be reintroduced into the donor, allowing for successful fertility[28]. This ideology could be transferred to female gametes and used as an infertility treatment there as well.
Heritable Diseases
The broad application of germline gene editing is to eliminate heritable, genetic diseases that would otherwise be fatal to a person's life. Parkinson's disease, cystic fibrosis, and spinal muscular atrophy are a few examples. Germline editing would eliminate the possibility that the at-risk individual would inherit the disease and pass the disease on to future generations. Preimplantation genetic diagnosis is currently the only treatment for heritable diseases like these. This procedure entails collecting at-risk embryos and selecting those that do not have the genetic mutation for implantation. Preimplantation genetic diagnosis contains flaws that germline genome editing does not. For example, if the inherited disease is dominant, most, if not all, embryos will have the disease. As a result, this method would be ineffective. Regardless of dominance status, germline gene editing can eliminate a heritable genetic disorder[29].
Beyond Ethical Concerns
Germline genomic editing has long-term cascading effects on the gene pool of the embryos whose genomes have been edited [30]. There is still a lot humans don't know about the extended effects of genome editing. One gene edited in a germline cell may have unintended consequences for future generations. Additionally, interactions between genes occur within a cell as many genes work together in pathways to perform a desired function. As a result, changing one gene may have an impact on the function of others. This concept of genetic interaction extends to the population level and is of concern given the rise of germline genome editing[31]. As this editing becomes more popular, more research is being conducted to analyze these effects and predict long-term safety.
References
Sources Cited:- ↑ Carroll, D. (2021) A short, idiosyncratic history of genome editing, Gene and Genome Editing. Elsevier. Available at: https://www.sciencedirect.com/science/article/pii/S2666388021000022
- ↑ “Full Stack Genome Engineering.” Synthego, https://www.synthego.com/learn/genome-engineering-history.
- ↑ “1972: First Recombinant DNA.” Genome.gov, https://www.genome.gov/25520302/online-education-kit-1972-first-recombinant-dna.
- ↑ Carroll, D. (2021) A short, idiosyncratic history of genome editing, Gene and Genome Editing. Elsevier. Available at: https://www.sciencedirect.com/science/article/pii/S2666388021000022
- ↑ CRISPR timeline (2018) Broad Institute. Available at: https://www.broadinstitute.org/what-broad/areas-focus/project-spotlight/crispr-timeline
- ↑ CRISPR timeline (2018) Broad Institute. Available at: https://www.broadinstitute.org/what-broad/areas-focus/project-spotlight/crispr-timeline
- ↑ Brooks, P.J. (2022) Somatic cell genome editing program, National Center for Advancing Translational Sciences. U.S. Department of Health and Human Services. Available at: https://ncats.nih.gov/somatic
- ↑ Bergman, M.T. (2022) Harvard researchers share views on future, ethics of gene editing, Harvard Gazette. Harvard Gazette. Available at: https://news.harvard.edu/gazette/story/2019/01/perspectives-on-gene-editing/
- ↑ Baylis, F. et al. (2020) Human Germline and heritable genome editing: The ... - The CRISPR Journal, Human Germline and Heritable Genome Editing: The Global Policy Landscape. Available at: https://www.liebertpub.com/doi/10.1089/crispr.2020.0082
- ↑ Bergman, M.T. (2022) Harvard researchers share views on future, ethics of gene editing, Harvard Gazette. Harvard Gazette. Available at: https://news.harvard.edu/gazette/story/2019/01/perspectives-on-gene-editing/
- ↑ Liu, S. (2020) Legal reflections on the case of genome-edited babies - global health research and policy, BioMed Central. BioMed Central. Available at: https://ghrp.biomedcentral.com/articles/10.1186/s41256-020-00153-4#:~:text=In%20the%20USA%2C%20Human%20genome,Institutes%20of%20Health%20(NIH).
- ↑ Liu, S. (2020) Legal reflections on the case of genome-edited babies - global health research and policy, BioMed Central. BioMed Central. Available at: https://ghrp.biomedcentral.com/articles/10.1186/s41256-020-00153-4#:~:text=In%20the%20USA%2C%20Human%20genome,Institutes%20of%20Health%20(NIH).
- ↑ Howard, H. and Niemiec, E. (2020) Ethical issues related to research on genome editing in human embryos, Computational and Structural Biotechnology Journal. Elsevier. Available at: https://www.sciencedirect.com/science/article/pii/S2001037019305173
- ↑ Paull, D. et al. (2012) Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants, Nature News. Nature Publishing Group. Available at: https://www.nature.com/articles/nature11800
- ↑ Emilia Niemiec, Heidi Carmen Howard, Ethical issues related to research on genome editing in human embryos, Computational and Structural Biotechnology Journal, Volume 18, 2020, Pages 887-896, ISSN 2001-0370, https://doi.org/10.1016/j.csbj.2020.03.014.
- ↑ Muigai, A.W.T. Expanding global access to genetic therapies. Nat Biotechnol 40, 20–21 (2022). https://doi.org/10.1038/s41587-021-01191-0
- ↑ Garver, KL, and B Garver. “Eugenics: Past, Present, and the Future.” American Journal of Human Genetics, U.S. National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/1928094/.
- ↑ Nicholas, Agar. “Why We Should Defend Gene Editing as Eugenics.” Cambridge Quarterly of Healthcare Ethics : CQ : the International Journal of Healthcare Ethics Committees, U.S. National Library of Medicine, 2019, https://pubmed.ncbi.nlm.nih.gov/30570459/.
- ↑ Nicholas, Agar. “Why We Should Defend Gene Editing as Eugenics.” Cambridge Quarterly of Healthcare Ethics : CQ : the International Journal of Healthcare Ethics Committees, U.S. National Library of Medicine, 2019, https://pubmed.ncbi.nlm.nih.gov/30570459/.
- ↑ Nicholas, Agar. “Why We Should Defend Gene Editing as Eugenics.” Cambridge Quarterly of Healthcare Ethics : CQ : the International Journal of Healthcare Ethics Committees, U.S. National Library of Medicine, 2019, https://pubmed.ncbi.nlm.nih.gov/30570459/.
- ↑ de Araujo, M. (2020) The ethics of genetic cognitive enhancement: Gene editing or embryo selection?, MDPI. Multidisciplinary Digital Publishing Institute. Available at: https://www.mdpi.com/2409-9287/5/3/20
- ↑ “What Are the Ethical Issues Surrounding Gene Therapy?: Medlineplus Genetics.” MedlinePlus, U.S. National Library of Medicine, 2022, https://medlineplus.gov/genetics/understanding/therapy/ethics/.
- ↑ Anomaly, Jonathan, et al. “Great Minds Think Different: Preserving Cognitive Diversity in an Age of Gene Editing.” Bioethics, U.S. National Library of Medicine, Jan. 2020, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6973122/.
- ↑ Anomaly, Jonathan, et al. “Great Minds Think Different: Preserving Cognitive Diversity in an Age of Gene Editing.” Bioethics, U.S. National Library of Medicine, Jan. 2020, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6973122/.
- ↑ Aggarwal, Ishani, et al. “The Impact of Cognitive Style Diversity on Implicit Learning in Teams.” Frontiers, Frontiers, 14 Jan. 2019, https://www.frontiersin.org/articles/10.3389/fpsyg.2019.00112/full.
- ↑ Aggarwal, Ishani, et al. “The Impact of Cognitive Style Diversity on Implicit Learning in Teams.” Frontiers, Frontiers, 14 Jan. 2019, https://www.frontiersin.org/articles/10.3389/fpsyg.2019.00112/full.
- ↑ Gyngell, Christopher. “Gene Editing and the Health of Future Generations.” Journal of the Royal Society of Medicine, U.S. National Library of Medicine, July 2017, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5524257/.
- ↑ Gyngell, Christopher. “Gene Editing and the Health of Future Generations.” Journal of the Royal Society of Medicine, U.S. National Library of Medicine, July 2017, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5524257/.
- ↑ Gyngell, Christopher. “Gene Editing and the Health of Future Generations.” Journal of the Royal Society of Medicine, U.S. National Library of Medicine, July 2017, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5524257/.
- ↑ Krekora-Zając, D. Civil liability for damages related to germline and embryo editing against the legal admissibility of gene editing. Palgrave Commun 6, 30 (2020). https://doi.org/10.1057/s41599-020-0399-2
- ↑ Rubeis, Giovanni, and Florian Steger. “Risks and Benefits of Human Germline Genome Editing: An Ethical Analysis.” Asian Bioethics Review, U.S. National Library of Medicine, 16 July 2018, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7747319/.