Gene Editing
Genome Editing, also known as gene editing, is a group of technologies under genetic engineering that gives scientists the ability to change an organism’s DNA. This group of technologies enable genetic material to be added, removed, or altered at particular locations in the genome. Several methods of implementing foreign DNA into an organism's existing genome have been utilized in the past, however, the most recent is called Crispr-Cas9. Short for clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9, the Crispr-Cas9 system has generated a lot of excitement among the scientific community as it has been the fastest, cheapest, most accurate and efficient means of altering DNA compared to its predecessors. Genome editing is of great interest in the prevention and treatment of human diseases. Currently, most research on genome editing is done to understand diseases using cells and animal models. Scientists are still working to determine whether this approach is safe and effective for use in people. It is being explored in research on a wide variety of diseases, including single-gene disorders such as cystic fibrosis, hemophilia, and sickle cell disease. It also holds promise for the treatment and prevention of more complex diseases, such as cancer, heart disease, mental illness, and human immunodeficiency virus (HIV) infection. [1]
Contents
History
The idea of using gene editing to treat disease or alter traits is not a novel one and dates back to at least the 1950s and the discovery of the double-helix structure of DNA. In the mid 20th century, researchers realized that small changes in the DNA sequence could mean the difference between health and disease. This understanding led to the theory that by identifying “molecular mistakes” that cause genetic diseases we could fix those mistakes, enabling the prevention or reversal of these diseases. This concept was the underlying idea behind gene therapy through genome editing, and was seen as the future of molecular genetics. [2]
Early Forms of Gene Editing
Zinc Finger Nucleases
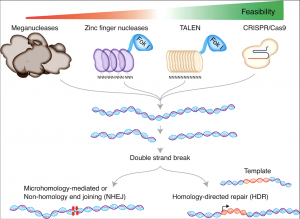
In 1985, possibly the first major technique, for highly targeted genome editing, was the discovery of zinc finger nucleases (ZFN), which improved the effectiveness of gene targeting. ZFNs help “recognize” DNA, which makes it possible to design ZFNs for a variety of genomic targets varying throughout different cells and organisms. [3] ZFNs are fusion proteins composed of DNA-binding domains that recognize and bind to a specific set of three base pairs. They can be combined to recognize longer DNA sequences. For example, specificity to a nine-base-pair target sequence would require three ZFN domains fused in tandem. [4] Researchers then fused the DNA cleaving (cutting) enzyme Fok1 to the ZFN binding domain to essentially create “genomic scissors” which can cleave DNA at a specified location, creating a double stranded break (DSB) in the DNA. [5] A repair template is then introduced, so a fraction of the cells will undergo homologous recombination, where the cell incorporates the gene of interest into its genome at the specific point. [6]
Transcription Activator-like Effector Nucleases
In 2007, the second generation of designer nucleases came in the form of transcription activator-like effector nucleases, or TALENs. These nucleases are able to recognize a single nucleotide rather than a trinucleotide motif like the ZFN. The TALENs benefit from ease of design, as effective TALENs can be designed and constructed in a few days, and can be multiplexed on the order of hundreds at a time. These nucleases work very similarly to ZFNs in that they are designed by fusing a DNA cutting domain of a nuclease to a TALE DNA recognition domain, which can be engineered to specifically recognize a unique sequence. These fusion proteins can then be used to form a targeted pair of “DNA scissors” that can be utilized to to perform targeted genome modifications such as sequence insertion, deletion, repair and replacement in living cells. TALENs offer 3 unique advantages compared to ZFNs. They have a higher DNA binding specificity, they have lower off-target effects, and they are easier to manufacture. [7]
Advances in Gene Editing
Although work on the CRISPR system began in the late 2000s, in 2012, official studies by Jennifer Doudna and Emmanuel Charpentier illuminated the biochemical mechanism of CRISPR technology and its utility in gene therapies. CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats, and the technology was engineered from a bacterial immune response present in nature. The machinery behind CRISPR works similarly to ZFNs and TALENs, however, unlike the prior gene editing technologies, CRISPR uses RNA-DNA binding, rather than protein-DNA binding, to guide nuclease activity, which enables application to a broader range of target sequences. Two seperate cleaving domains have been used and are in use. These are the cas9 and cpf1 domains. Each has their own unique benefits and shortcomings, including producibility and applicability. [8]
Use of Gene Editing
Treating Disease
Leukemia
In 2015, scientists successfully used somatic gene therapy when a one-year old in the United Kingdom named Layla received a gene editing treatment to help her fight leukemia, a type of cancer. These scientists did not use CRISPR to treat Layla, and instead used another genome editing technology called TALENs. Doctors tried many treatments before this, but none of them seemed to work, so scientists received special permission to treat Layla using gene therapy. This therapy ended up working and she made a full recovery. [9]
CRISPR Baby
In 2018, He Jiankui a chinese scientist, at a conference in Hong Kong, announced that he had created genetically modified twin sisters. He utilized the CRISPR-Cas9 editing procedure to rewrite DNA in the girls’ embryos to modify the twins genetic makeup to be more resistant to certain diseases. He claimed that the modifications in the twins’ DNA would make them immune to the Human Immunodeficiency Virus (HIV) infection by turning a gene called CCR5 into a mutant form that prevents the virus from invading cells. [10] However, these claims have yet to be substantiated and further tests must be conducted to see whether any off-target mutations also took place or any other unforeseen complications could arise for the twins in the future. [11]
Agriculture
Ethical Issues
External Links
Further Reading
References
- ↑ "What Are Genome Editing and CRISPR-Cas9?" NIH.” U.S. National Library of Medicine, National Institutes of Health, 3 Mar. 2020.
- ↑ Fridovich-Keil, Judith L. "Gene Editing" Encyclopædia Britannica, Inc., 20 Feb. 2020
- ↑ “Full Stack Genome Engineering.” Synthego,
- ↑ Fridovich-Keil, Judith L. "Gene Editing" Encyclopædia Britannica, Inc., 20 Feb. 2020
- ↑ “Zinc Finger Nuclease" Wikipedia, Wikimedia Foundation, 29 Jan. 2020,
- ↑ “Full Stack Genome Engineering.” Synthego,
- ↑ “Genome Editing.” Wikipedia, Wikimedia Foundation, 25 Feb. 2020,
- ↑ Fatemeh Safari, Khadijeh Zare, Manica Negahdaripour, Mazyar Barekati-Mowahed & Younes Ghasem "CRISPR Cpf1 proteins: structure, function and implications for genome editing" Cell & Bioscience, BioMed Central, 1 Jan. 1970,
- ↑ “What Is Genome Editing?”
- ↑ Sample, Ian "Chinese scientist who edited babies' genes jailed for three years" The Guardian, Guardian News and Media, 31 Dec. 2019
- ↑ Cyranoski, David "The CRISPR-Baby Scandal: What's next for Human Gene-Editing." Nature News, Nature Publishing Group, 26 Feb. 2019