Genetically Modified Babies ("Designer Babies")

Once a science-fiction concept and now a reality, a genetically-modified embryo, commonly referred to as a “designer baby,” is defined as a baby that has been genetically engineered in order to include or remove certain genes associated with a certain disease or phenotype.[1] This process can be completed in two different ways. The more common process involves pre-implantation genetic diagnosis (PGD), which according to Johns Hopkins University, is reproductive technology that is made up of “a screening test that can be performed on embryos created via in vitro fertilization (IVF) to genetically analyze the embryos prior to transfer.”[2] The second and less common method is genome editing which involves actually modifying the embryos’ DNA sequences. This was first done by Chinese researcher He Jiankui in November of 2018 on a pair of twins in order to protect them against human immunodeficiency virus (HIV).[3] Although successful, He’s Youtube announcement about the procedure during the International Summit on Human Genome Editing in Hong Kong stunned the world. Some scientists were thrilled about the potential benefits, yet others within the international scientific community raised a myriad of ethical concerns.[4] Due to the widespread concern and controversy, He Jiankui was sentenced to three years in prison and fined 3 million yuan, or $430,000 U.S. dollars, for “illegal medical practice.” According to the court, they claimed that it was primarily problematic because his practices were too premature and put the children at risk.[5]
As outlined below, some of the major concerns regarding genetic engineering embryos include the following: lack of embryo permission, potential financial discrimination, aesthetic use, potentially harmful generational imprint, and dangerously quick release due to lack of regulation. Many of the ethical concerns revolve around the definition of an embryo and consequently, whether they legally have all human rights, similar to the debate on abortion versus pro-life.[6] Given the existing controversies, there still is no general consensus from the public and more specifically, from the scientific community, about how genomic editing will affect society moving forward.
Contents
How They Work
Pre-implantation Genetic Diagnosis
The first process, PGD, requires in vitro fertilization in order to acquire the embryos that will be used for the procedure. Once the eggs have been retrieved and fertilized, medical professionals perform a procedure known as assisted hatching. This is done in order to obtain cells which will be biopsied and analyzed in order to scan for specific genetic traits, such as mutated cells.[7] Then, only embryos with the desired traits, such as those lacking genetic diseases, are transferred to the uterus to initiate the process of pregnancy.[8]
Genomic Editing
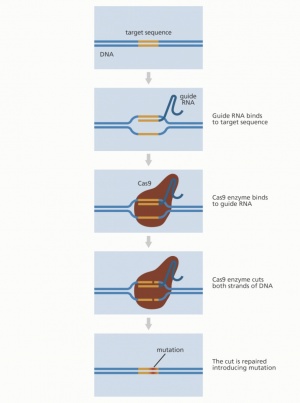
As noted before, the second process, genomic editing, requires editing the embryos’ DNA sequences with cutting and deletion. This method been used by researchers since the 1970’s to edit the genomes of different organisms, such as yeast and bacteria, in order to learn more about genomic sequencing and how it affects humans. [9]The first genome editing technologies were created in the late 20th century, with the discovery of restriction enzymes, which identify patterns within nucleotide sequences and cut at specific spots in order to insert new DNA material. Then, in the 1980s, zinc finger nucleases (ZFNs) were discovered, which could achieve the same thing through a similar process, but with higher accuracy and efficiency due to the way in which they increase the length of the DNA recognition site and thus minimize error. Then, the use of transcription activator-like effector nucleases (TALENS) emerged in 2011. They are similar to ZFNs, but also more accurate and efficient. However, to use them was time and cost ineffective, which led scientists to continue searching for new genomic editing options.[10] The most recent and rapidly developing genome editing tool used to edit humans is known as CRISPR (clustered regularly interspaced short palindromic repeats). [11] Invented in 2009 by Dr. Jennifer Doudna and Dr. Emmanuelle Charpentier, this tool is much more time and cost-efficient as well as more accurate than any previous method. It has been used to develop two different gene therapies: germline therapy and somatic therapy. Germline therapy alters the DNA in sperm and eggs, in other words, reproductive cells and consequently, can change reproductive cells in future generations. On the contrary, somatic therapies target non-reproductive cells, so these changes are not passed down from generation to generation. [12]
Uses
There are several known uses for utilizing PGD and genome editing. It is primarily used to prevent genetic diseases by identifying abnormal or known disease-related chromosomes and then selecting against them. While all couples may consider the procedure, couples who tend to use it in order to achieve pregnancy and avoid a genetic disease include parents who carry an heritable disease or who have had difficulties getting pregnant in the past. For example, women over 38 years old and men with sperm abnormalities have a higher probability of producing embryos with chromosomal mutations.[13] A few diseases that could potentially be prevented include Huntington’s disease, cystic fibrosis, forms of Alzheimer’s disease, types of breast and ovarian cancer, myotonic dystrophy, beta-thalassaemia and many others. All of these diseases are currently associated with premature death, severe chronic disability, or other critical consequences.[14]
Another known use for the procedure is to select embryos of a specific sex. This is most commonly done by couples when an heritable disease is strongly associated with one sex over another, such as the X-linked disorder haemophilia. Some couples may also choose the sex of their child due to preference although this has become a subject of controversy.[15]For example, currently gender selection is legal in the United States, but not in other countries, such as Canada.[16] Regarding cost and risk involved, according to Legacy IVF, a specialized fertility services clinic, “The average cost of gender selection using PGD/PGS is approximately $3,000 to $5,000” and it does not pose any threats to the mother or child.[17]
Other uses include the ability to potentially detect viruses. For example, cancer researchers have found that it can detect DNA from cancer-causing viruses and RNA from cancer cells.[18] It has specifically been proven that it can identify DNA related to human papillomavirus (HPV), specifically HPV types 16 and 18, in extractions from cultured human cells.[19] In addition, recent experiments have also shown that it may be able to detect coronavirus.[20]
Regulation
PGD use is currently only allowed in certain countries. Within the United States, rules regarding PGD use depend on the jurisdiction of each state. In many countries, PGD is not allowed in any territory. Some of these countries include, but are not limited to, Chile, Switzerland, China and Austria.[21] In other countries, PGD is permitted, but only for certain circumstances, such as for screening of specific diseases. For example, in Spain, France and Sweden, PGD must comply with proper practice and regulations.[22]
Ethical Considerations
While there are known scientific benefits to using gene therapies in order to select specific embryos or modify their DNA, there are also a variety of ethical considerations that have led the topic of "designer babies" to be very controversial:
Lack of Embryo Consent
Currently three definitions that define a human embryo, including the following: “(1) The embryo is a human person, having an inalienable right to life; (2) The embryo is a heap of cells with the same moral status as the one of other cells and therefore, we behave toward it as it is a thing or a property; (3) The embryo is not a person, owner of rights, but it has to be protected as it is a potential person or a special entity.”[23] Depending on one’s perspective, their opinion changes about what rights a human embryo should have. Those that believe an embryo is the equivalent of a human person believe that they deserve all human rights. One concern about genetically modified or selected babies is that the embryo is unable to give permission as to whether or not they want their genetics altered or specifically chosen. Those that have this concern believe that the embryo has the same rights as a fully grown human.[24] On the other hand, other perspectives believe that the embryo is “a heap of cells” or “is not a person” and thus does not have autonomy over itself.[25] People with this belief, including some bioethicists that believe that prenatal autonomy should grant parents the right to decide the fate of their children, including their gender or selected traits. Many scientists who take this stance have pointed out, “embryos at the pre-14 day stage are simply incapable of giving consent or exercising any form of choice” so this logic holds true for all decisions made by the parent prior to this point.[26] Others have also argued that the embryo cannot have a legal status because it does not have the capability to regulate its own nature because it cannot comprehend what itself is. For this reason, these people argue that it does not need to give consent.[27]
Financial Discrimination
Another concern is that the process of PGD or genome editing is very expensive for some based on their lifestyle and income. According to Pacific Fertility Center Los Angeles, PGD can cost anywhere from $4,000 to $10,000. This does not include the cost of IVF, the procedure that takes place when PGD is normally performed, which ranges from $10,000 to $30,000. These costs also do not include if the parents are using a third-party surrogate or egg donor, which would create additional costs. Another major factor that could influence cost is the amount of IVF cycles needed. All of these prices vary slightly among all clinics, but according to Pacific Fertility Center Los Angeles, they align with the standard price for legal use of these procedures.[28]
Consequently, many believe that these procedures may increase the wealth gap if only a small percentage of the population can afford it. This could lead to greater health disparities if only the extremely wealthy are able to select against genetic disorders. It could also lead to different types of discrimination if only those who can afford to use these procedures can select the traditionally most desirable traits for their future children. This specifically is a major concern of UNESCO’s International Bioethics Committee (IBC), which wrote: “It weakens the idea that the differences among human beings, regardless of the measure of their endowment, are exactly what the recognition of their equality presupposes and therefore protects. It introduces the risk of new forms of discrimination and stigmatization for those who cannot afford such enhancement or simply do not want to resort to it.”[29] This has then led people to question where the line between therapy and enhancement lies. [30]
Aesthetic Use
One other concern about genetically modified or selected babies is that many believe that using these processes for the specific purpose of selecting for desirable aesthetic traits, including sex, is unethical. This is where the term "designer baby" comes from. Those that do not support gene therapies for this purpose typically believe that visual appearance should be up to fate, not the parents of a baby, especially if it will affect generations to come. Marcy Darnovsky, an American policy advocate who directs the Center for Genetics and Society, told National Public Radio (NPR), “The scenario is that you would have fertility clinics advertising to people who wanted to engineer their future children so that they could be presented as 'enhanced' — as biologically better than everyone else.” She continues, “It's not a world we want to build. It's not a world we want to live in."[31]
Sex selection is currently not considered ethical in many countries because many people believe that it could be used to perpetuate sexist beliefs and behavior. For this reason, sex selection is currently banned in Canada, Taiwan and the United Kingdom.[32]
Studies and conventions have been held internationally over the past few years in order to gauge public concern about this specific ethical consideration. For example, in a study published by Nature Biotechnology in 2017, a group of researchers led a study in order to find out the public consensus on the potential application of genomic editing on embryos. They conducted online surveys including over 1,000 participants from Austria, Hungary, Iceland, Italy, the Netherlands, Portugal, Spain, UK and the United States in order to gather opinions about gene editing. They provided two contexts including “therapy (curing a disease” and “enhancement (improving memory and learning capacity” and two recipients “adult” and “prenatal.” The results showed 75% of the comments were positive for adult therapy whereas for adult enhancement, only 26% of the comments were positive. Furthermore, prenatal therapy had more positive comments in comparison to adult enhancement although still lesser than adult therapy. Prenatal enhancement had drastically negative comments, reflecting that use of genomic editing for cosmetic uses is a major ethical concern for the general public internationally.[33]
Generational Imprint
Another controversial aspect of these selective genetic procedures is that changes can be inherited by generations to come rather than just the immediate individual affected. Similar to the way in which the affected individuals cannot give permission to undergo the procedure given that they are simply embryos at the time, future generations also cannot give permission to be impacted by these changes since they have not even been conceived yet. As one NPR article notes, “on a broader level, any changes made in the DNA of an embryo would be passed down for generations. That raises fears that any mistakes in the editing that inadvertently caused new diseases could become a permanent part of that family's genetic blueprint.”[34] For example, some doctors believe that that if CRISPR edits the wrong locations of a gene, unintended consequences could occur. One doctor, Alejandro Chaxez, M.D., Ph.D., an assistant professor at Columbia University, has states that “If [CRISPR] starts breaking random parts of the genome, the cell can start stitching things together in really weird ways, and there’s some concern about that becoming cancer.”[35] For this reason, a multitude of countries and organizations do not support germline editing and have regulations in place against it. For example, the National Institutes of Health (NIH) has not approved funding for this research. Due to safety, ethical and policy concerns, the NIH states on its website: “NIH does not fund any use of genome editing in human embryos. The Dickey-Wicker amendment prohibits the use of Congressionally appropriated funds for the creation of human embryos for research purposes or for research in which human embryos are destroyed.”[36]
On the other hand, generational imprint is also a direct reason that some researchers support this technology. For example, Dr. Paula Amato, an associate professor of obstetrics and gynecology at Oregon Health and Science University in Portland who worked on the international team of scientists who first successfully edited human embryos, claims that “Anytime there's a new technology there's a potential for misuse. We have to acknowledge that.” They added, “Personally I don't feel that's a reason not to pursue the research if you think there's a potential benefit that outweighs that risk. And I think if you can prevent serious disease in future generations, that makes it worthwhile to pursue this."[37]
Release and Regulation
One final concern is related to not necessarily the technology, but rather the speed at which it is being released due to the social, cultural, and health implications it may have on society. In 2017, the National Academy of Sciences and the National Academy of Medicine released a report that stated “Genome editing holds great promise for preventing, ameliorating, or eliminating many human diseases and conditions,” but “Along with this promise, however, comes the need for ethically responsible research and clinical use.”[38] One of their key recommendations note that the following principles must underline all future research: promoting well-being; transparency; due care; responsible science; respect for persons; fairness; transnational cooperation. Until these principles are met, human genome-editing research and product approval cannot continue. Many institutions and researchers agree with this philosophy, believing that the technology is far too powerful to rush its release and that strict regulation must first be developed and then enforced.[39] As Fredrik Lanner, a geneticist at Karolinska Institutet in Stockholm, notes, “I think this needs to be tightly regulated. This is very exciting. But it also could be a double-edged sword. So I think we really have to be extra cautious with this technology."[40] This ethical consideration outlines people's concern about its regulation and impact rather than concern about the technology existing and being used in itself.
References
- ↑ Ly, S. (2011, March 31). Ethics of Designer Babies | The Embryo Project Encyclopedia. The Embryo Project Encyclopedia. Retrieved January 23, 2022, from https://embryo.asu.edu/pages/ethics-designer-babies
- ↑ Preimplantation Genetic Testing (PGD) | The Johns Hopkins Fertility Center. (n.d.). Johns Hopkins Medicine. Retrieved January 23, 2022, from https://www.hopkinsmedicine.org/gynecology_obstetrics/specialty_areas/fertility-center/infertility-services/preimplantation-genetic-testing.html
- ↑ Greely, H. T. (2019, October). CRISPR’d babies: human germline genome editing in the ‘He Jiankui affair’. Journal of Law and the Biosciences, 6(1), 111-183. US National Library of Medicine National Institutes of Health. 10.1093/jlb/lsz010
- ↑ van Beers, B. C. (2020, June 9). Rewriting the human genome, rewriting human rights law? Human rights, human dignity, and human germline modification in the CRISPR era. Journal of Law and the Biosciences, 7(1). https://doi.org/10.1093/jlb/lsaa006
- ↑ Cyranoski, D. (2020, January 3). What CRISPR-baby prison sentences mean for research. nature. Retrieved February 9, 2022, from https://www.nature.com/articles/d41586-020-00001-y
- ↑ Aluas, M., Gherman, C. D., & Dumitrescu, C. I. (2017). Is the human embryo legally defined and protected? Causes and consequences. Romanian Journal of Morphology & Embryology, 58(2), 695-700.
- ↑ Preimplantation Genetic Testing (PGD) | The Johns Hopkins Fertility Center. (n.d.). Johns Hopkins Medicine. Retrieved January 23, 2022, from https://www.hopkinsmedicine.org/gynecology_obstetrics/specialty_areas/fertility-center/infertility-services/preimplantation-genetic-testing.html
- ↑ What is PGD? (n.d.). Genetics & IVF Institute. Retrieved January 23, 2022, from https://www.givf.com/geneticservices/whatispgd.shtml
- ↑ How Does Genome Editing Work? (2017, August 3). National Human Genome Research Institute. Retrieved January 23, 2022, from https://www.genome.gov/about-genomics/policy-issues/Genome-Editing/How-genome-editing-works
- ↑ Mah, A. (2019, May 1). Genome Editing Techniques: The Tools That Enable Scientists to Alter the Genetic Code. Synthego. Retrieved February 11, 2022, from https://www.synthego.com/blog/genome-editing-techniques#4-gene-editing-techniques-tools-to-change-the-genome
- ↑ Hsu, P. D., Lander, E. S., & Zhang, F. (2014, June 05). Development and Applications of CRISPR-Cas9 for Genome Engineering. US National Library of Medicine National Institutes of Health, 157(6), 1262-1278. 10.1016/j.cell.2014.05.010
- ↑ What is genome editing? (2019, August 15). National Human Genome Research Institute. Retrieved January 23, 2022, from https://www.genome.gov/about-genomics/policy-issues/what-is-Genome-Editing
- ↑ What is PGD? (n.d.). Genetics & IVF Institute. Retrieved January 23, 2022, from https://www.givf.com/geneticservices/whatispgd.shtml
- ↑ Preimplantation Genetic Diagnosis (PGD). (n.d.). Fertility Treatment Abroad. Retrieved February 10, 2022, from https://fertility.treatmentabroad.com/tests-and-investigations/preimplantation-genetic-diagnosis-pgd
- ↑ How Is Gender Selection Performed Using PGD. (n.d.). New York Fertility Institute. Retrieved January 23, 2022, from https://www.nyfertility.org/blog/how-is-gender-selection-performed-using-pgd
- ↑ What Is Gender Selection and Who Is a Good Candidate? (n.d.). Legacy IVF. Retrieved February 11, 2022, from https://legacyivf.com/gender-selection/
- ↑ What Is Gender Selection and Who Is a Good Candidate? (n.d.). Legacy IVF. Retrieved February 11, 2022, from https://legacyivf.com/gender-selection/
- ↑ National Cancer Institute Staff. (2020, July 27). How CRISPR Is Changing Cancer Research and Treatment. National Cancer Institute. Retrieved February 9, 2022, from https://www.cancer.gov/news-events/cancer-currents-blog/2020/crispr-cancer-research-treatment
- ↑ Chen, J. S., Ma, E., Harrington, L. B., Da Costa, M., Tian, X., Palefsky, J. M., & Doudna, J. A. (2018, April 27). CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science, 360(6387), 436-439. National Center for Biotechnology Information. 10.1126/science.aar6245
- ↑ National Cancer Institute Staff. (2020, July 27). How CRISPR Is Changing Cancer Research and Treatment. National Cancer Institute. Retrieved February 9, 2022, from https://www.cancer.gov/news-events/cancer-currents-blog/2020/crispr-cancer-research-treatment
- ↑ Preimplantation Genetic Diagnosis (PGD). (n.d.). Fertility Treatment Abroad. Retrieved February 10, 2022, from https://fertility.treatmentabroad.com/tests-and-investigations/preimplantation-genetic-diagnosis-pgd
- ↑ Preimplantation Genetic Diagnosis (PGD). (n.d.). Fertility Treatment Abroad. Retrieved February 10, 2022, from https://fertility.treatmentabroad.com/tests-and-investigations/preimplantation-genetic-diagnosis-pgd
- ↑ Aluas, M., Gherman, C. D., & Dumitrescu, C. I. (2017). Is the human embryo legally defined and protected? Causes and consequences. Romanian Journal of Morphology & Embryology, 58(2), 695-700.
- ↑ Fineberg, H., & Chan, S. (n.d.). The ethics of changing genes in the embryo | Eurostemcell. EuroStemCell. Retrieved February 10, 2022, from https://www.eurostemcell.org/ethics-changing-genes-embryo
- ↑ Aluas, M., Gherman, C. D., & Dumitrescu, C. I. (2017). Is the human embryo legally defined and protected? Causes and consequences. Romanian Journal of Morphology & Embryology, 58(2), 695-700.
- ↑ Fineberg, H., & Chan, S. (n.d.). The ethics of changing genes in the embryo | Eurostemcell. EuroStemCell. Retrieved February 10, 2022, from https://www.eurostemcell.org/ethics-changing-genes-embryo
- ↑ Aluas, M., Gherman, C. D., & Dumitrescu, C. I. (2017). Is the human embryo legally defined and protected? Causes and consequences. Romanian Journal of Morphology & Embryology, 58(2), 695-700.
- ↑ PGS/PGT-A testing costs and candidates. (2021, February 2). Pacific Fertility Center of Los Angeles. Retrieved February 11, 2022, from https://www.pfcla.com/blog/pgs-pgta-testing-costs
- ↑ International Bioethics Committee. (2008). Report of the International Bioethics Committee of UNESCO (IBC) on consent. UNESCO. Retrieved February 9, 2022, from https://unesdoc.unesco.org/ark:/48223/pf0000178124
- ↑ van Beers, B. C. (2020, June 9). Rewriting the human genome, rewriting human rights law? Human rights, human dignity, and human germline modification in the CRISPR era. Journal of Law and the Biosciences, 7(1). https://doi.org/10.1093/jlb/lsaa006
- ↑ Stein, R. (2017, August 2). Scientists Able To Fix Disease Gene In Experimental Embryos : Shots - Health News. NPR. https://www.npr.org/sections/health-shots/2017/08/02/540975224/scientists-precisely-edit-dna-in-human-embryos-to-fix-a-disease-gene
- ↑ Preimplantation Genetic Diagnosis (PGD). (n.d.). Fertility Treatment Abroad. Retrieved February 10, 2022, from https://fertility.treatmentabroad.com/tests-and-investigations/preimplantation-genetic-diagnosis-pgd
- ↑ Gaskell, G., Bard, I., Allansdottir, A., da Cunha, R. V., Eduard, P., Hampel, J., Hildt, E., Hofmaier, C., Kronberger, N., Laursen, S., Meijknecht, A., Nordal, S., Quintanilha, A., Revuelta, G., Saladié, N., Sándor, J., Santos, J. B., Seyringer, S., Singh, I., … Zwart, H. (2017, November 9). Public views on gene editing and its uses. Nature Biotechnology, 35, 1021-1023. https://doi.org/10.1038/nbt.3958
- ↑ Stein, R. (2017, August 2). Scientists Able To Fix Disease Gene In Experimental Embryos : Shots - Health News. NPR. https://www.npr.org/sections/health-shots/2017/08/02/540975224/scientists-precisely-edit-dna-in-human-embryos-to-fix-a-disease-gene
- ↑ National Cancer Institute Staff. (2020, July 27). How CRISPR Is Changing Cancer Research and Treatment. National Cancer Institute. Retrieved February 9, 2022, from https://www.cancer.gov/news-events/cancer-currents-blog/2020/crispr-cancer-research-treatment
- ↑ Gene Editing – Digital Media Kit. (n.d.). National Institutes of Health (NIH). Retrieved February 10, 2022, from https://www.nih.gov/news-events/gene-editing-digital-press-kit
- ↑ Stein, R. (2017, August 2). Scientists Able To Fix Disease Gene In Experimental Embryos : Shots - Health News. NPR. https://www.npr.org/sections/health-shots/2017/08/02/540975224/scientists-precisely-edit-dna-in-human-embryos-to-fix-a-disease-gene
- ↑ National Academies of Sciences, Engineering, and Medicine. (2017). Oversight of Human Genome Editing and Overarching Principles for Governance1. In Human Genome Editing: Science, Ethics, and Governance. National Academies Press. https://doi.org/10.17226/24623
- ↑ National Academies of Sciences, Engineering, and Medicine. (2017). Oversight of Human Genome Editing and Overarching Principles for Governance1. In Human Genome Editing: Science, Ethics, and Governance. National Academies Press. https://doi.org/10.17226/24623
- ↑ Stein, R. (2017, August 2). Scientists Able To Fix Disease Gene In Experimental Embryos : Shots - Health News. NPR. https://www.npr.org/sections/health-shots/2017/08/02/540975224/scientists-precisely-edit-dna-in-human-embryos-to-fix-a-disease-gene